An Extractables and Leachables (E&L) study forms part of the testing process which enables pharmaceutical companies to market their devices and/or drug products. The E&L process assesses whether there are any harmful leachables which could have a detrimental impact upon the safety or efficacy of the drug product.
There are numerous potential sources for leachable contaminants, including: process equipment, primary packaging, label adhesive, printing inks or secondary packaging.
The E&L assessment is extremely important for ensuring patient protection and is included as part of the drug’s documentation to gain regulatory approval. E&L testing assesses whether contaminants migrate from the process equipment, device (delivery system) material or packaging into the drug product. The basic requirements have been around for over 80 years and it is only in the last 10-15 years that a lot more detail has come out about the requirements:
Then:
Food, Drug and Cosmetic Act - Section 501(a)(3)
“…a drug is deemed to be adulterated if its container is composed, in whole or part, of any poisonous or deleterious substance which may render the contents injurious to health…” (1938)
Now:
21 CFR Part 211.94 (a)
“Drug product containers and closures shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality or purity beyond the official or established requirements.”
21 CFR Part 211.65
“Equipment shall be constructed so that surfaces that contact components, in-process materials, or drug products shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements.”
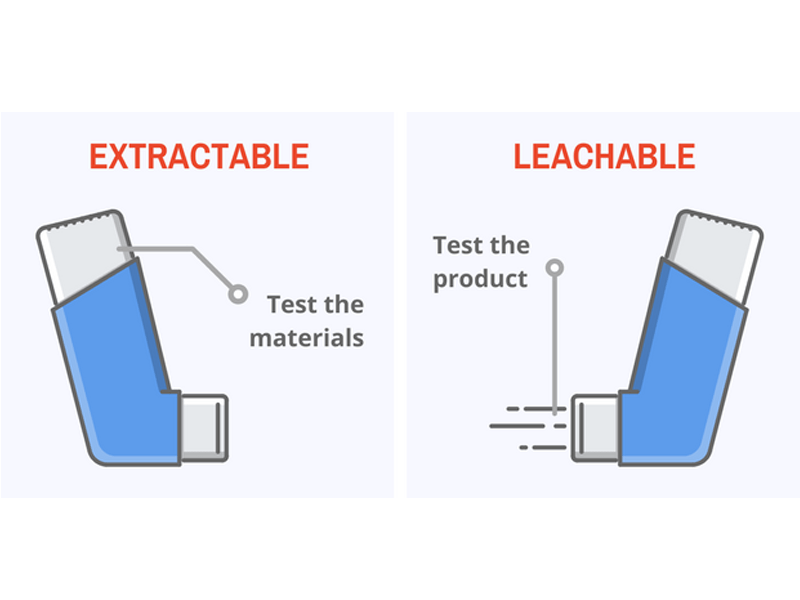
'Extractable' vs 'Leachable'
Extractables are the chemical species which can be released from a material under exaggerated conditions, such as temperature, solvent etc. Extractable testing allows the material to be assessed to see if any potential harmful leachables could be produced and potentially identify if a different material is required to be selected. Not all extractables end up as leachables.
Leachables are chemicals that migrate from the primary or secondary container closure system or the manufacturing system into the drug product under normal conditions or during accelerated studies and are exposed to the patient.
Leachable testing requires the analysis of the final product up to and including end of shelf life. This life time assessment allows the actual impact of the leachables found. Leachables, having a potential direct toxicological impact to patient safety, are one of the critical quality attribute of concern for regulators.
The regulatory landscape
There are numerous global organisations that have recommendations for the execution of an E&L testing program, including: regulators such as FDA, EMEA, Health Canada etc., other organizations such as USP,EP, ASTM, ICH, industry consortia ELSIE, IPAC-RS BPSA, BPOG, and scientific and academic groups PQRI, PDA and AAPS.
Test programs
There is not a one-size-fits-all test design and strategy. The impact of leachables depends on a number of factors, and these affect how a study is planned, including:
How the pharmaceutical is delivered to the patient e.g. inhalation, injection, oral, topical etc.
The dosing regime - is it once in a lifetime, or multiple times per day for the rest of the patient’s life, etc.
For extractables study design, these factors affect how a study is designed with regards to solvent selection, sample preparation techniques, analytical methods and specifying limits of detection.
Typical analytical techniques for extractables and leachables studies (E&L) include:
- Gas Chromatography Mass Spectrometry (GC-MS)
- Liquid Chromatography Mass Spectrometry (LC-MS/UV)
- Static Headspace Gas Chromatography Mass Spectrometry (SH GC-MS)
- Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES).
Find out more about the
extractables and leachables testing services that Smithers offer.
.png?ext=.png)