Differences Between ISO 13485 and ISO 9001
We compare the similarities and differences between ISO 13485 and ISO 9001
In the competitive landscape of the medical device industry, achieving ISO 13485 certification is not just an option but a necessity for organizations seeking to uphold operational excellence. We specialize in delivering top-tier ISO 13485 audit services, expertly crafted to align with the rigorous standards set forth by the International Organization for Standardization (ISO). Our services are tailored specifically to the medical device sector, ensuring that your organization not only meets but exceeds the compliance expectations required to thrive in this critical industry.
By choosing our ISO 13485 audit services, you gain not only a partner committed to regulatory compliance but also a catalyst for continuous improvement and operational excellence. We help you build and maintain trust with stakeholders, regulatory bodies, and consumers alike. Our track record in the medical device sector speaks for itself, showcasing our dedication to helping organizations achieve seamless certification and sustained success.
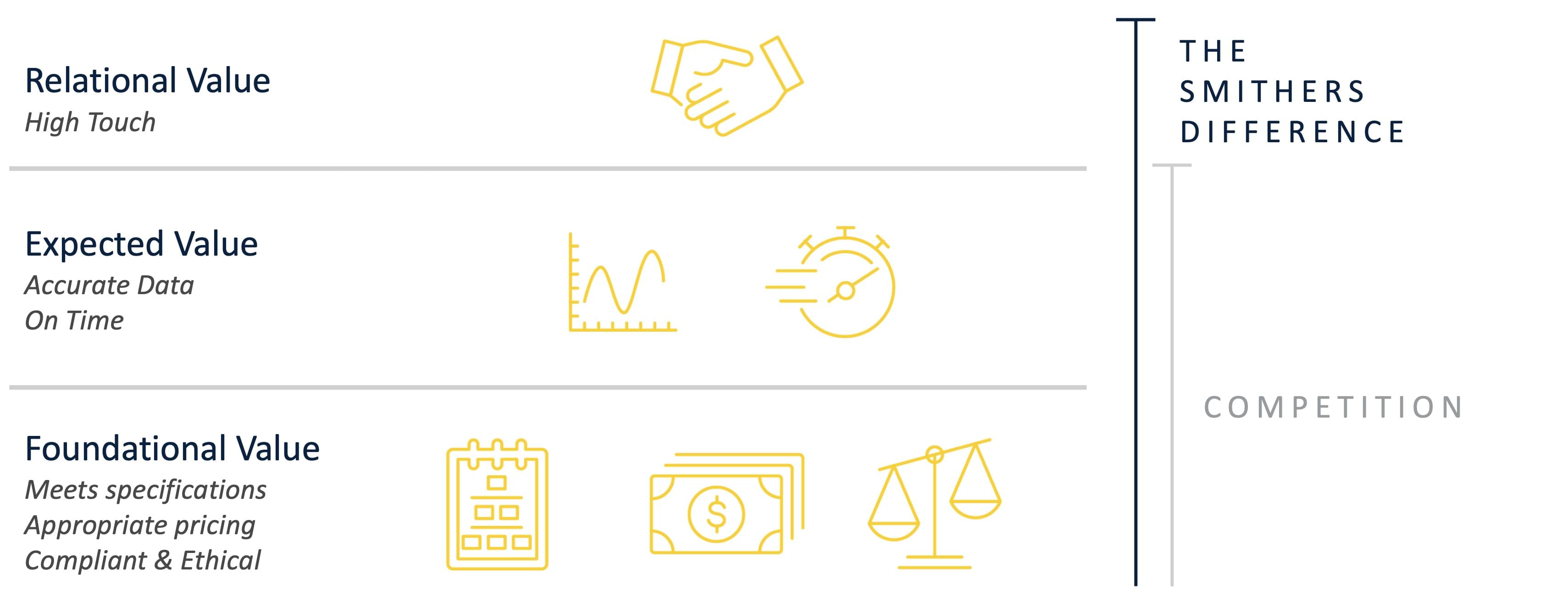
Smithers believes there is a very basic foundational value when offering third-party assessments. Often, other certification bodies struggle to satisfy even the first level. Meanwhile, we consistently strive to achieve the 3rd level by:
Our ISO 13485 audit services are designed for medical device organizations, including but not limited to:
Achieving ISO 13485 certification is more than just compliance; it’s a commitment to excellence, safety, and performance in the medical device industry. Partner with us to achieve certification with confidence.
ISO 13485 is a globally recognized Quality Management System (QMS) standard designed to meet the rigorous requirements of the medical device industry.
Certification proves your organization’s commitment to high-quality, compliant processes, increasing credibility, operational harmony, and competitive advantage.
Timelines vary depending on your organization’s readiness, but our streamlined approach accelerates the process while maintaining meticulous attention to detail.
Yes. Our experts offer ongoing post-certification support to help you maintain compliance and focus on continual improvement.